Kinetics: The Chemical Industry's Kryptonite
How kinetics ruins control, leads to undesirable outcomes, and makes chemical production look like a casino.
At some point in every chemical engineer’s education, the term “unit operation” will start to pop up everywhere, and everyone sort of acts like they know what it means—it’s those individual blocks on process flow diagrams, right?
The “unit operations” idea really comes down to this: if you look at any industrial process with granularity, you’ll find that many seemingly different processes in different sectors use the same unit of operation (e.g. a reactor, distillation column, heat exchanger, etc.).
The taxonomy was pioneered by Arthur Dehon Little, an American chemical engineer who, in a speech given at MIT in 1916, said:
“Any chemical process, on whatever scale conducted, may be resolved into a coordinate series of what may be termed “unit operations”, as pulverising, dyeing, roasting, crystallising, filtering, evaporation, electrolysing and so on.”
But Little’s terminology doesn’t have to be confined to a chemical engineer’s education. All we’re really talking about here is the transformation of inputs into outputs at the equipment (aka unit) level instead of at the process level.
The process level is usually easy to understand. And some processes, like assembly, are even obvious from an outsider’s perspective: car parts go into a factory, and cars go out the factory. There’s only one possible outcome, an assembled car, and so nobody is confused about what vehicle manufacturers do in their factories.
Ultimately, this is all about the ability to control the outcomes of a given process.
If you trace your way back from assembled car to the steel and plastic that it’s made of, the operations we need to do shift from macro-level transformations to micro-level transformations. And as we shift in that direction, we simultaneously lose control. That’s why chemical companies make so many by-products and co-products. It’s like trying to assemble F-150s, but routinely ending up with Miatas.
Dealing with unavoidable and undesirable outcomes is not uncommon in other industries. Think about how a casino might operate:
Individuals enter and begin to wander around. Those individuals then encounter games, food, and entertainment that lead to different outcomes. For example, that individual encounters a slot machine, takes a seat, which then results in one of two outcomes: either the casino makes money, or the casino loses money.
Everyone knows that the house eventually wins, but this is especially true for slots:
“Local and regional casinos remain focused primarily on slot machines, which still account for about 70% of their gross revenues and an even higher percentage of their profits” — Clyde Barrow
Given this profitability, the casino must exert at least some control of the outcomes here. But how much control does the casino really have over its most profitable “unit operation”?
In truth, slots are highly profitable for a lot of reasons we won’t get into, but most principal among them is their ability to control a) how likely an individual is to sit down at a slot machine, and b) how likely a slot machine is to make the individual lose money.
Specifically, casinos can influence how likely an individual is to sit down at a slot machine by a) serving alcohol, b) teasing people by making it obvious when someone wins big, or c) by squeezing as many slot machines in the building as possible. Further, a casino can influence how likely a slot machine is to make an individual lose money by changing the programming of the machines.
It’s a similar story for chemical producers. Recall how individuals entered the casino—instead, molecules enter a reactor and begin to bounce around. Sometimes those molecules bump into each other, and sometimes they bump into each other hard enough to make a reaction happen.
So if making the reaction happen is the desirable outcome, how much can chemical producers control a) the likelihood of molecular impact, and b) the likelihood that molecular impact results in a reaction?
While inebriating and psychological tactics don’t work for chemical producers, they do have their own tricks. A chemical producer can make molecular impact more likely by a) altering the concentration of molecules in the reactor, or by b) giving the molecules a dedicated place to react, like on a catalyst. And just like a casino, a chemical producer can influence how likely that molecular impact results in a reaction by increasing the temperature of the reactor.
But what would happen if the chemical producer increased the temperature of the reactor? What would happen if the casino changed the slot machine programming so that every spin at a slot machine makes them money?
Casinos might see very high profits for a couple of days, but that might deter people from coming back. Similarly, chemical producers could operate a reactor at 2000°C, but that might make some other reaction occur.
In other words, neither casinos or chemical producers can achieve their desired outcome without simultaneously realizing undesirable outcomes.
And it gets worse! There are far more potential outcomes at casinos than just winning or losing slots, and there are far more potential outcomes in reactors than a couple of possible reactions.
Other stuff happens at casinos. You watch your buddy lose money for an hour. You go to the bathroom. You win a few hundred play blackjack.
Chemical producers are plagued by similar outcome variation. Your two reactants might be able to react in 3 different ways, each of which produces different products at different rates, of which some of those products might then react with either one of your two initial reactants.
So, what can we make of this? Why do these undesired outcomes occur?
If you read my last post, you should have taken away one core idea from thermodynamics: while some reactions release energy, most reactions practiced by industry require energy. Today we’re adding kinetics to the mix.
The first thing you need to understand is that even though some reactions release energy, they still need some energy to get started.
That amount of energy is called the activation energy. It’s not entirely accurate, but you can think of it as the amount of energy needed to break the bonds of a molecule in a reasonable amount of time. That energy requirement is why wood doesn’t spontaneously combust and why diamonds don’t suddenly become graphite (even though both would release energy).
The second thing you need to understand is a little more nuanced. When you have multiple potential reactions, speed matters.
Let’s say you’re an ambitious chap who wants to figure out how we can utilize CO2. Initially you might think that it’s hopeless because CO2 sits at the bottom of the oxidation ladder, but I have great news—you don’t have to climb up the ladder the same way you climbed down.
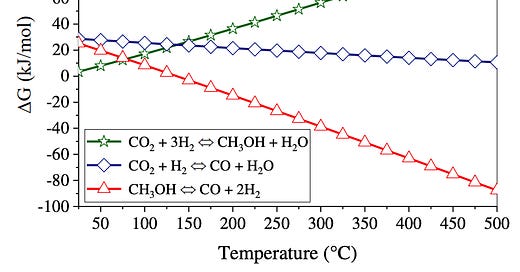
Take CO2 hydrogenation for example. Assume that three reactions are possible (the three lines), and that the only thing we can control is the temperature (x-axis) of the reactor. The y-axis is analogous to movement along an oxidation ladder: if it’s positive, the reaction requires energy (a step up the ladder), and if it’s a negative, the reaction releases energy (a step down the ladder).
The reaction we want to happen, the synthesis of methanol, is represented by the green line. Okay, now look at where it intercepts the y-axis. The value is slightly positive—just a small step up the oxidation ladder. So, you go to the lab, you mix up some CO2 and hydrogen, and what do you find? Surprise! It’s not methanol.
That’s because thermodynamics (i.e. what the oxidation ladder illustrates) isn’t enough to predict what reactions will actually happen. The oxidation ladder fails to ask how much more likely is it that one CO2 molecule slams into one hydrogen molecule than how likely it is for one CO2 molecule to have a four-way collision with three other hydrogen molecules. (Hint: the one-on-one collision is more likely.)
Chemical production isn’t just burdened by inherently energy-intensive unit operations. It’s also burdened by an inability to control the outcomes of the micro-scale transformations.
So, what can we do about it? Can we eliminate that activation energy? Can we get better at controlling which reactions happen?
Improving catalysis gets at the root of both the activation energy and control issues, but good separations processes effectively does the same thing—it just requires more capital, results in higher operational costs, and leads to enormous complexes like BASF’s site in Ludwigshafen.
Maybe we’ll talk about that next time.





Enzymes!!!!!!!!
What are your favourite science books for beginners?